



业务咨询
中国:
Email: marketing@medicilon.com.cn
业务咨询专线:400-780-8018
(仅限服务咨询,其他事宜请拨打川沙总部电话)
川沙总部电话: +86 (21) 5859-1500
海外:
+1(781)535-1428(U.S.)
0044 7790 816 954 (Europe)
Email:marketing@medicilon.com




Jellies offer a palatable alternative to traditional tablets, improving medication adherence in children and the elderly. A study comparing different formulations of common cold medicines (syrup, jellies, and tablets) revealed that while none were bioequivalent according to FDA standards, jellies and syrup exhibited similar absorption rates and extents. Tablets, however, significantly delayed and reduced drug absorption compared to the syrup. These findings suggest that jellies can be a patient-friendly formulation with comparable bioavailability to syrup.
To evaluate the effect of jelly formulation on the bioavailability of cold medicines, two types of jellies were prepared for a fixed-dose combination of acetaminophen (AAP), chlorpheniramine maleate (CPM), dextromethorphan hydrobromide (DMH), and dl-methylephedrine hydrochloride (MEH).
Pharmacokinetic Study in Beagle Dogs
A pharmacokinetic study was performed in beagle dogs after oral administration in Medicilon Preclinical Research (Shanghai) LLC (Shanghai, China).
The plasma concentration profiles of AAP, CPM, DMH, and MEH are presented in the figure below. As shown in the initial plasma concentration profile for 4 h, the jellies and syrup showed rapid absorption of AAP, while both tablets retarded the absorption of AAP exhibiting lower Cmax and delayed Tmax.
The jellies had less delay in the absorption rate and better bioavailability than the tablets.
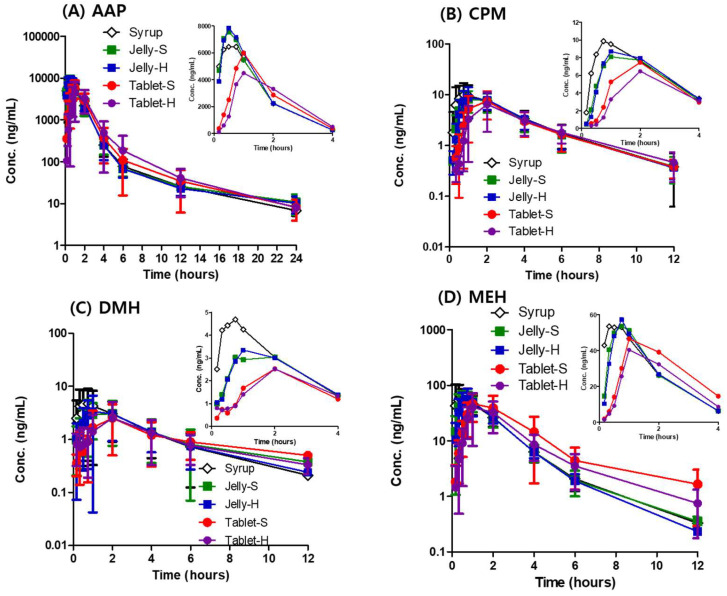
Plasma concentration-time profile of (A) AAP, (B) CPM, (C) DMH, and (D) MEH in dogs after oral administration of various formulations at a dose of 150, 1.875, 18.75, and 11.25 mg, respectively. Each point represents mean ± standard deviation (n=15).
Reference:
 相关新闻
相关新闻